Physics for Electrical Engineering: Unit III: Semiconductors and Transport Physics
Energy Band Diagram
intrinsic semiconductor
At very low temperature say 0K, no free electrons are available for conduction. Hence, this semiconductor behaves as an insulator at very low temperature.
ENERGY BAND DIAGRAM
Fig.
3.3 shows a two-dimensional crystal structure of germanium and energy band
representation of intrinsic semiconductor at very low temperature.
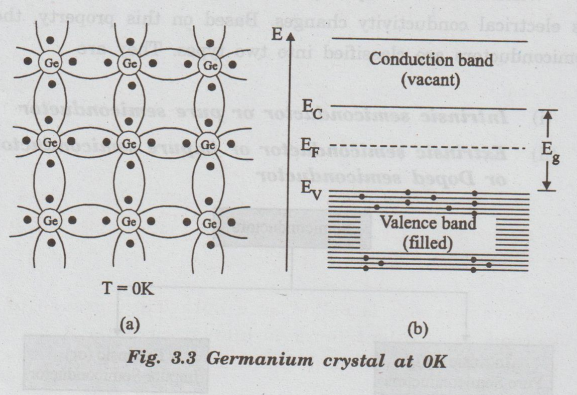
Fig.
3.3(a) Two-dimensional representation of germanium solid. divibuba No free
electron is available as all the valence electrons are engaged in covalent
bonds.
Fig.
3.3(b) Energy band representation. Valence band is fully ni nost occupied and
conduction band is completely vacant.
At
very low temperature say 0K, no free electrons are available for conduction.
Hence, this semiconductor behaves as an insulator at very low temperature.
Charge carriers in intrinsic semiconductor
To
get free electrons, covalent bonds must be broken. There are many ways of
breaking covalent bond and setting the electrons free. One such way is to increase
crystal temperature above 0K.
When
the temperature of intrinsic semiconductor is increased, some of the electrons
get sufficient energy to break covalent bonds.
Once
the electrons are liberated from bond, they become free electrons. These free
electrons move randomly through crystal. (Fig. 3.4(a))
As
shown in fig. 3.4 (b), the energy required to break a covalent bond and to set
an electron free is equal to band gap energy Eg. It is about 0.72 eV
for germanium and 1.1 eV for (d) silicon.
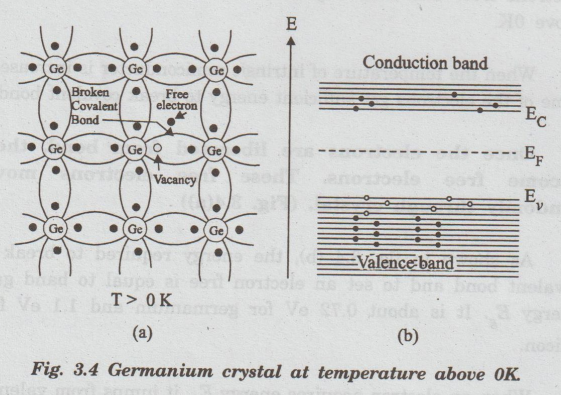
When
an electron acquires energy E, it jumps from valence 'g' band to conduction
band. As a result, a vacant site (empty space) is created in valence band.
This
vacant site is called as a hole. The absence of an electron in covalent bond is
known as hole. A hole can attract an electron and hence it acts as a positive
charge.
When
an electrical field is applied, these free electrons acquire directional motion
and contribute to electrical conductivity.
For
every electron freed from covalent bond, one hole is created in the crystal. It
is relatively easy for a valence electron in a neighbouring atom to leave its
covalent bond and fill this hole.
As
a result, an electron moving from a covalent bond to fill a hole leaves behind
a hole in its original position.
The
hole effectively moves in a direction opposite to that of an electron. The hole
in its new position may now be filled by an electron from another covalent
bond.
Thus hole will correspondingly move one more step in the direction opposite to the motion of the electron.
(a)
Thermal vibrations of atoms lead to breaking up of covalent bonds.
Consequently, a free electron and a vacancy are produced simultaneously.
(b)
Energy band representation. Energy E (=E-E) causes elod transition of electrons
from valence band to conduction evithe band, leaving vacancies (hole) behind.
Therefore,
in intrinsic semiconductor, current conduction is due to the movement of both
electrons and holes.
Here,
the number of electrons is equal to the number of holes at any given
temperature.
Physics for Electrical Engineering: Unit III: Semiconductors and Transport Physics : Tag: : intrinsic semiconductor - Energy Band Diagram
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
