Physics for Electrical Engineering: Unit III: Semiconductors and Transport Physics
Extrinsic or Impure Semiconductors
Definition, Advantages, Types
In a semiconducting material, if the charge carriers originate from impurity atoms which are doped to the original material, then this type of semiconductor is known as extrinsic or impure semiconductor.
EXTRINSIC OR IMPURE SEMICONDUCTORS
In
a semiconducting material, if the charge carriers originate from impurity atoms
which are doped to the original material, then this type of semiconductor is
known as extrinsic or impure semiconductor.
It
is also known as doped semiconductor.
Extrinsic
semiconductor is obtained by adding trivalent or pentavalent impurity atoms to
a tetravalent semiconductor. The electrical properties of pure semiconductors
can be easily changed even with the addition of very little amount of
impurities.
Doping
The
addition of impurities to a pure semiconductor is known as doping and added
impurity is called as doping agent or dopant.
The
addition of impurities increases the number of free electrons and holes in
semiconductor and hence increases its electrical conductivity.
Some
of the common doping agents are arsenic, antimony, phosphorus, gallium,
aluminium and boron. These elements have either five or three valence electrons
in the outermost orbit.
Advantages
of Extrinsic semiconductors
•
Electrical conductivity is high.
•
Electrical conductivity can be altered to any desired value by controlling of
doping concentration.
•
Electrical conductivity is not a function of temperature.
Types of Extrinsic semiconductors
The
extrinsic semiconductors are classified into two types based on the type of
impurity added. (Fig 3.6)
(i)
n - type semiconductor
(ii)
p - type semiconductor
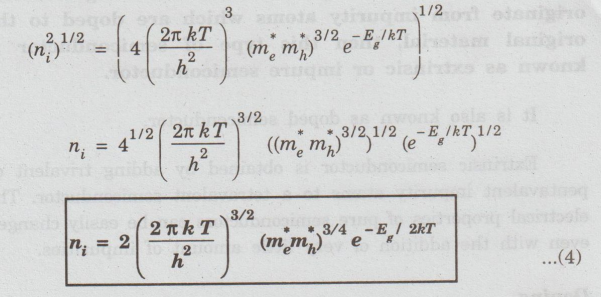
n-type
semiconductor
When
a small amount of pentavalent impurity (group V element) is doped to a pure
semiconductor, it becomes n-type semiconductor.
Such
impurities are known as donor impurities because they donate free
electrons to semiconductor crystal.
Typical
examples of pentavalent impurities are phosphorus, (Atomic No. 15) and antimony
(Atomic No. 51).
Covalent
bond in n-type semiconductor
A
pentavalent impurity (phosphorus) having five valence electrons is added to a
pure semiconductor having four valence electrons (silicon or germanium).
Now,
four electrons of germanium form a covalent bond with four valence
electrons of phosphorus (impurity atom).
The
fifth electron which is now free finds no place in covalent bond structure as
shown in fig. 3.7 (a).
We
have one electron left free. This acts as a conduction electron. A very small
amount of energy (0.01 eV for germanium and 0.05 eV for silicon) is needed to
detach this fifth electron.
The
addition of pentavalent impurity gives a large number of free electrons
(negative charges) in semiconductor. Therefore, it is called n semiconductor
where n stands for negative type.
Since
every pentavalent atom contributes one free electron, in addition to thermally
generated electron-hole pairs, the number of free electrons is more than the
number of holes in n-type semiconductor.
Thus
in this case, electrons are majority charge carriers and holes are minority
charge carriers.
Energy
band of n-type semiconductor
The
energy band diagram of n-type semiconductor is shown in fig.3.7 (b). When the
donor impurities are added, the allowable energy levels (donor energy levels)
are introduced.
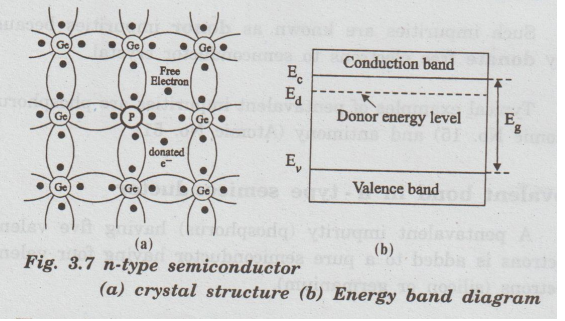
These
donor energy levels are slightly below the conduction band. They are discrete
and do not form a band because the impurity atoms are far away in the crystal
and hence their interaction is small.
The
donor energy level for germanium is 0.01 eV and for silicon it is 0.05 eV below
the conduction band. Therefore, even at room temperature, almost all the fifth
electrons enter into the conduction band.
Physics for Electrical Engineering: Unit III: Semiconductors and Transport Physics : Tag: : Definition, Advantages, Types - Extrinsic or Impure Semiconductors
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
