Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation
Ionic Polarisation
Polarization mechanisms in dielectrics
Ionic polarisation is due to the displacement of cations (positive ions) and anions (negative ions) in opposite directions.
IONIC
POLARISATION
Ionic
polarisation is due to the displacement of cations (positive ions) and anions
(negative ions) in opposite directions.
This occurs in ionic dielectrics (e.g. NaCl
crystal) by the influence of external electrical field (fig. 1.6 (a)).

When
an electrical field (E) is applied on an ionic dielectric, there is a shift of
one ion with respect to another from their mean positions.
The
positive ions displace in the direction of applied electrical field through the
distance x1. The negative ions displace in opposite direction
through the distance x2 (fig. 1.6 (b)).

We
assume that there is one cation and one anion in each unit cell of the ionic
crystal.
Hence,
the net distance between two ions
x
= x1 + x2.................. (1)
When
the ions are displaced from their mean positions in their respective
directions, then the restoring forces appear which tend to move the ions to
move back to their mean position. The restoring force produced is proportional
to the displacement.
For positive ion
Restoring
force F ∝ x1
or
Restoring force acting on the positive ion F = β1x1 ...
(2)
For negative ion
Restoring
force F ∝ x2
or
Restoring force acting on the negative ion F = β2 X2....(3)
where
β1, and β2 are restoring force constants which depend
upon the masses of ions and angular frequency of vibrating molecule in which
ions are present.
If
m is the mass of positive ion, M is the mass of negative ion and ω0 is the angular frequency, then
β1
= mω02........(4)
β2
= Mω02.......(5)
Substituting
for β1, from eqn (4) in eqn (2), the restoring force for positive
ion can be rewritten as
F
= mω02 x1 ..............(6)
We
know that force F = eE .......(7)
Equating
the eqn (6) and (7), we get
eE
= mω02 x1
or
x1 =eE / m ω02 ...(8)
Similarly
for the negative ion, we can write
X2
= eE / Mω02 .....(9)
Adding
the equations (8) and (9), we have
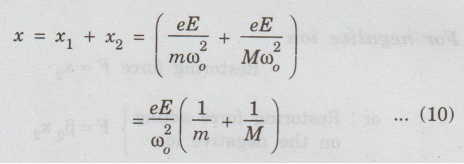
The
dipole moment is equal to the product of charge and distance of separation
between the charges.
i.e.,
μ = e × x ...(11)
Substituting
x from eqn (10) in eqn (11), we have
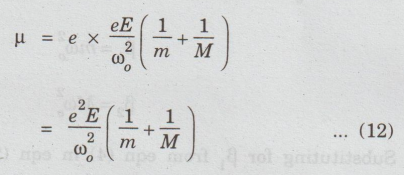
But
μ α Ε
Or
μ = αi Ε.....(13)
where
αi is ionic polarisability of dielectric material
On
comparing the equations (12) and (13), we have
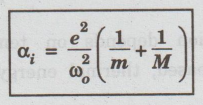
Conclusion
(i) Ionic polarisability (∝i)
is inversely proportional to the square of angular frequency of the ionic
molecule.
(ii)It
is directly proportional to its reduced mass given by (1/ m + 1/M )
(iii)
It is independent of temperature.
(iv)
It occurs in ionic substance.
Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation : Tag: : Polarization mechanisms in dielectrics - Ionic Polarisation
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
