Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation
Orientational Polarisation
Polarization mechanisms in dielectrics
Orientational polarisation takes place only in polar dielectrics. Polar dielectrics have molecules with permanent dipole moments even in the absence of an electrical field as shown in fig. 1.7.
ORIENTATIONAL
POLARISATION
Orientational
polarisation takes place only in polar dielectrics. Polar dielectrics have
molecules with permanent dipole moments even in the absence of an electrical
field as shown in fig. 1.7.
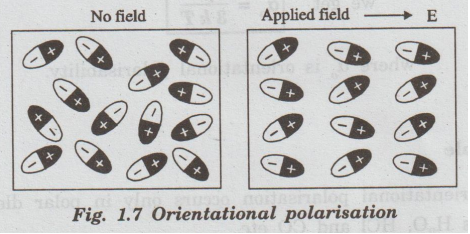
When
the polar dielectrics are subjected to an electric field, the molecular dipoles
are oriented in the direction of electric field.
The
contribution to polarisation due to orientation of molecular dipoles is called
orientational polarisation.
This
polarisation depends on temperature. When the temperature is increased, thermal
energy tends to disturb the alignment.
From
the Langevin's theory of paramagnetism, net intensity isnolinogorq Nu2B
of
magnetisation = N μ2B / 3kT
Since
the same principle can be applied to the application of electric field in
dielectrics, we may write
Orientational
polarisation
P0
= N μ2E / 3kT ...(1)
But,
orientational polarisation is proportional to applied field  and it is
given by
and it is
given by
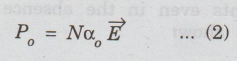
Comparing
the equations (1) and (2),
we
get α0 = μ2 / 3kT
where
∝0
is orientational polarisability.
Example
Orientational
polarisation occurs only in polar dielectrics such as H2O, HCl and
CO etc.
Conclusion
The
orientational polarisability is inversely proportional to absolute temperature
of the material.
Note
Orientational polarisation occurs only in polar dielectrics ie.,
dielectric with molecules having permanent dipoles.
Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation : Tag: : Polarization mechanisms in dielectrics - Orientational Polarisation
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
