Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation
Introduction to Insulation breakdown in gases, liquids and solids
In practical applications, the failure or breakdown of a dielectric material is of grat concern to engineers. There are different mechanisms by which dielectric breakdown takes place.
INTRODUCTION
TO INSULATION BREAKDOWN IN GASES, LIQUIDS AND SOLIDS
In
practical applications, the failure or breakdown of a dielectric material is of
grat concern to engineers. There are different mechanisms by which dielectric
breakdown takes place.
INSULATION BREAKDOWN IN GASEOUS
DIELECTRICS
The
breakdown in gaseous dielectrics is due to ionization caused by collision of
electrons. When a strong electric field is applied the accelerated free
electrons act energy greater than the ionization of the gas.
The
breakdown is initiated with a spark discharge. Then this develops to a arc
discharge. It results in a high current density and direct short circuit. The
dielectric strength of gaseous dielectrics depends on
(i)
Pressure: The dielectric strength is
higher at very high and at very low pressure.
(ii)
Uniformly of applied electric field: In
non-uniform 26g boqq electric fields to the field intensity tends to
concentrate in certain points. This stress concentration initiate collision
ionization at relatively low voltage.
(iii) Polarity of electrodes: The breakdown
voltage deb depends on the polarity of electrodes. Thus if point and plain
electrodes are used, the breakdown voltage will gabe higher when the point
electrode is negative.
(iv)
Frequency of applied field: When the frequency is increased the breakdown
voltage decreases. But, breakdown voltage increases after reaching a minimum
value at about 1 MHz.
(v)Distance
between the electrodes.
(vi)Chemical
composition of the gas.
DIELECTRIC BREAKDOWN IN LIQUIDS
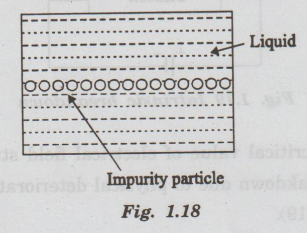
In
impure liquids there are small conductive particles in suspension and coalesce
end to end. Thus, impurity particles form a conducting bridge between the
electrodes. This leads to discharge.
•
In some liquids, the discharge initiates as partial discharges in gas bubbles
entrapped in the liquid. These partial discharges can raise the temperature and
vaporize more of the liquid. (Fig. 1.17)
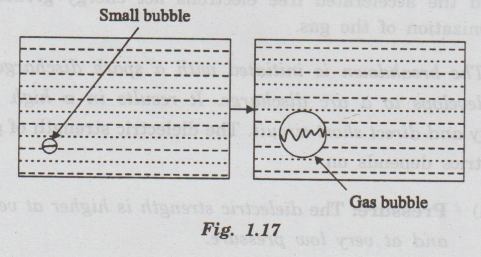
Thus
the size of the bubble increases. The eventual discharge can be a series of
partial discharges in entrapped gas bubbles.
•
Moisture absorption and absorption of gases generally bas nio deteriorate the
dielectric strength. The oxidation of lliw oge certain liquids, such as oils,
produces more acidic and hence higher conductivity eventually give discharge.
•
In some liquids, the discharge involve the emission of a large number of
electrons from the electrode into the liquid due to field emission at high
fields. This is a discharge process by electrode injection.
DIELECTRIC BREAKDOWN SOLIDS
There
are various major mechanisms that can lead to dielectric breakdown in solids.
The most likely mechanism depends on the dielectric material's condition and
sometimes on extrinsic factors such as the ambient conditions, moisture
absorption being a typical example.
Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation : Tag: : - Introduction to Insulation breakdown in gases, liquids and solids
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
